N2 Molar Mass: A Deep Dive Into Nitrogen's Building Blocks
Ever wondered what makes the air we breathe so unique? Well, buckle up because we're diving headfirst into the world of nitrogen and its molar mass. N2 molar mass might sound like a mouthful, but trust me, it's a fascinating topic that affects everything around us. Whether you're a science enthusiast or just curious about the basics, this article will break it down for you in a way that's both informative and engaging.
Now, let's get one thing straight: nitrogen is everywhere. It makes up about 78% of the Earth's atmosphere, which means it's kind of a big deal. Understanding its molar mass helps us grasp how this essential element behaves in different situations. So, whether you're studying chemistry, engineering, or just want to sound smart at your next dinner party, knowing about N2 molar mass is a game-changer.
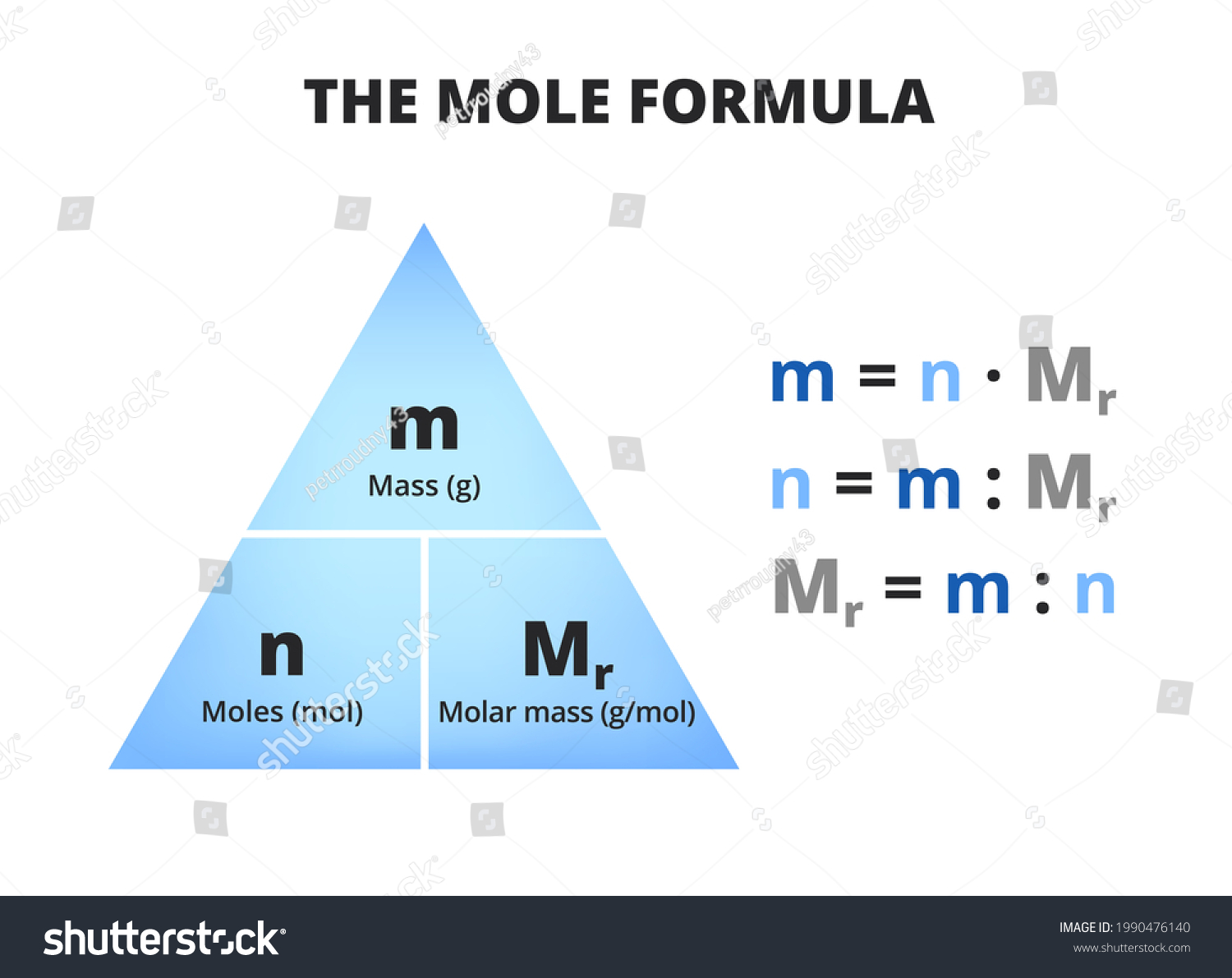
Before we dive deeper, let's set the stage. Molar mass is essentially the weight of one mole of a substance, and for nitrogen, it's no different. The N2 molar mass plays a crucial role in various scientific calculations, from determining gas volumes to understanding chemical reactions. So, let's unravel the mystery together and see why this topic deserves your attention.
- Unlocking The Secrets Of Surveyspanoramaedkipp Texaslogin A Comprehensive Guide
- 1000000 Yen Dollars Unlocking The Power Of Currency Exchange And Wealth
What Exactly is N2 Molar Mass?
Alright, let's start with the basics. N2 molar mass refers to the mass of one mole of nitrogen gas (N2). To put it simply, it's the weight of 6.022 x 10^23 nitrogen molecules. Now, if you're scratching your head wondering why this matters, let me tell you—it's crucial for scientists, engineers, and even environmentalists. Knowing the molar mass of nitrogen allows us to calculate things like gas density, reaction rates, and even predict how gases behave under different conditions.
The molar mass of nitrogen is approximately 28.014 g/mol. This number comes from adding the atomic masses of two nitrogen atoms since nitrogen exists as a diatomic molecule (N2). It's like saying, "Hey, two nitrogen atoms hanging out together weigh about 28 grams if we gather 6.022 x 10^23 of them." Pretty neat, right?
Why Does Molar Mass Matter?
Here's the deal: molar mass isn't just some random number in a chemistry textbook. It's a key player in understanding how substances interact. For instance, knowing the N2 molar mass helps us figure out how much nitrogen is needed for industrial processes like ammonia production or even in the production of fertilizers. Plus, it's vital in atmospheric studies where scientists monitor nitrogen levels to understand climate change and air quality.
- How Tall Is Jeff Goldblum Discover The Real Facts About The Iconic Star
- Unstoppable Force The Coach Of Baltimore Ravens Leading The Pack
Imagine being able to calculate how much nitrogen is in the air you breathe or how much is needed to inflate a car tire. That's the power of understanding molar mass. It bridges the gap between theoretical knowledge and real-world applications.
The Science Behind N2 Molar Mass
Now that we've got the basics down, let's delve into the science. The concept of molar mass is rooted in the mole, a fundamental unit in chemistry. A mole is like a counting unit, similar to how we use dozens to count eggs. But instead of 12, a mole represents 6.022 x 10^23 particles, known as Avogadro's number. This is where things get interesting.
When it comes to nitrogen, the molar mass is calculated by adding the atomic masses of two nitrogen atoms. Each nitrogen atom has an atomic mass of approximately 14.007 g/mol, so when you double that, you get the N2 molar mass of 28.014 g/mol. It's like putting two puzzle pieces together to form a complete picture.
How is Molar Mass Measured?
Measuring molar mass involves some pretty cool techniques. One common method is mass spectrometry, where scientists zap molecules with a beam of electrons to break them apart and measure their mass. Another method is using the ideal gas law, which relates pressure, volume, temperature, and the number of moles of a gas. By knowing the volume and pressure of a gas, scientists can calculate its molar mass.
For nitrogen, these methods confirm the theoretical value of 28.014 g/mol. It's like double-checking your math homework to make sure you didn't miss anything. Scientists rely on these precise measurements to ensure accuracy in their research and applications.
Applications of N2 Molar Mass
Now that we've cracked the science behind N2 molar mass, let's talk applications. Nitrogen's molar mass is crucial in various industries and fields. For starters, it's essential in the production of ammonia, a key ingredient in fertilizers. Farmers around the world depend on nitrogen-based fertilizers to boost crop yields, and understanding its molar mass helps optimize this process.
Beyond agriculture, nitrogen is used in the electronics industry to create inert atmospheres during manufacturing. It's also vital in the food industry, where nitrogen is used to preserve perishable items by displacing oxygen. In the medical field, nitrogen is used in cryopreservation to store biological samples at extremely low temperatures.
Environmental Impact
Understanding N2 molar mass also plays a role in environmental studies. Nitrogen is a major component of the atmosphere, but its excessive release into the environment can lead to issues like eutrophication and air pollution. By monitoring nitrogen levels and understanding its behavior, scientists can develop strategies to mitigate these environmental impacts.
For example, knowing the molar mass of nitrogen helps in calculating emissions from industrial processes and vehicles. This data is crucial for policymakers to set regulations and guidelines to protect our planet. It's like having a tool to measure the health of our environment and take action when needed.
Historical Context of Nitrogen
Let's take a trip back in time to understand how nitrogen was discovered and its significance. Nitrogen was first isolated by Daniel Rutherford in 1772, who called it "noxious air." Back then, scientists were just beginning to understand the composition of air, and nitrogen's discovery was a major breakthrough. Fast forward to today, and nitrogen is one of the most studied elements in science.
The concept of molar mass itself has evolved over the years. Avogadro's hypothesis, proposed in the early 19th century, laid the foundation for understanding molecular weights. This hypothesis stated that equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. It was a game-changer in the field of chemistry and helped scientists calculate molar masses more accurately.
Key Milestones
Throughout history, several milestones have shaped our understanding of nitrogen and its molar mass. The development of the periodic table by Dmitri Mendeleev in the 1860s provided a systematic way to organize elements based on their atomic masses. Later, advancements in analytical techniques like mass spectrometry allowed scientists to measure molar masses with unprecedented precision.
These milestones highlight the importance of continuous research and innovation in science. They remind us that our understanding of the world is constantly evolving, and every discovery builds on the knowledge of those who came before us.
Common Misconceptions About N2 Molar Mass
Let's clear up some common misconceptions about N2 molar mass. First off, some people think that nitrogen's molar mass is exactly 28 g/mol. While it's close, the actual value is 28.014 g/mol. This small difference might seem insignificant, but in scientific calculations, precision matters. It's like the difference between hitting a bullseye and being just a little off.
Another misconception is that molar mass only applies to gases. In reality, molar mass is a fundamental property of all substances, whether they're in solid, liquid, or gas form. Understanding this helps scientists apply the concept across different states of matter.
Why Precision Matters
Precision in molar mass calculations is crucial for several reasons. In pharmaceuticals, for example, even a small error in molar mass can lead to incorrect dosages. In environmental studies, precise measurements help scientists accurately model atmospheric conditions. It's like having a ruler with fine gradations instead of a rough estimate. The more precise your measurements, the better your results.
So, the next time someone tells you nitrogen's molar mass is exactly 28, you can confidently correct them and explain why precision matters in science. It's all about getting it right, down to the last decimal point.
Real-World Examples of N2 Molar Mass
Let's bring it back to real-world examples to see how N2 molar mass is applied in everyday life. For instance, in the automotive industry, nitrogen is used to inflate tires. Knowing the molar mass of nitrogen helps engineers calculate the right pressure and volume needed for optimal tire performance. It's like giving your car a little extra boost without even realizing it.
In the food industry, nitrogen is used to package snacks like chips and crackers. The molar mass of nitrogen helps determine the amount needed to displace oxygen and prevent spoilage. It's why your chips stay fresh for longer and don't turn stale as quickly.
Fun Facts
Did you know that nitrogen is also used in liquid form for cryopreservation? Liquid nitrogen, which has a boiling point of -196°C, is used to freeze biological samples for long-term storage. The molar mass of nitrogen plays a role in calculating the amount needed for this process. It's like having a super-cold freezer that can preserve anything from cells to organs.
Another fun fact is that nitrogen is used in maglev trains to create a frictionless surface. By using nitrogen gas to levitate the train, engineers can achieve high speeds with minimal resistance. It's like floating on air, but with science.
Future Research and Developments
As science continues to advance, so does our understanding of nitrogen and its molar mass. Researchers are exploring new ways to use nitrogen in sustainable energy solutions, such as nitrogen-based batteries. These batteries have the potential to store energy more efficiently and reduce our reliance on fossil fuels.
Additionally, scientists are studying nitrogen's role in climate change and how it interacts with other atmospheric gases. Understanding these interactions can help develop strategies to mitigate global warming and protect our planet. It's like putting together a giant puzzle where every piece matters.
What's Next?
The future of nitrogen research looks promising. With advancements in technology and analytical techniques, scientists can measure molar masses with even greater precision. This opens up new possibilities for applications in medicine, engineering, and environmental science. It's like having a toolbox with better and better tools to solve complex problems.
So, whether it's improving crop yields, developing sustainable energy solutions, or understanding climate change, nitrogen and its molar mass will continue to play a vital role in shaping our future. The possibilities are endless, and the journey is just beginning.
Conclusion
As we wrap up our deep dive into N2 molar mass, it's clear that this topic is much more than just a number. It's a gateway to understanding the world around us and how nitrogen plays a crucial role in various aspects of life. From agriculture to industry, and from environmental science to everyday applications, N2 molar mass is a cornerstone of scientific knowledge.
So, the next time you take a deep breath, remember that nitrogen is there, quietly doing its job. And if you're ever asked about N2 molar mass, you'll be able to confidently explain its significance and applications. Now, here's your call to action: share this article with a friend, leave a comment with your thoughts, or explore more articles on our site. Together, let's keep the conversation going and continue learning about the amazing world of science.
Table of Contents
- What Exactly is N2 Molar Mass?
- The Science Behind N2 Molar Mass
- Applications of N2 Molar Mass
- Historical Context of Nitrogen
- Common Misconceptions About N2 Molar Mass
- Real-World Examples of N2 Molar Mass
- Future Research and Developments
- Conclusion
- Unveiling The Mysteries Of Johnny From Hotel Transylvania The Ultimate Fan Guide
- Lil Yachty Real Name Unveiling The Rise Of A Hiphop Sensation
3,595 Molar Mass Images, Stock Photos & Vectors Shutterstock

How do you Calculate the Molar Mass of a Substance? Write A Topic

The Mole Molar Mass And Molar Volume vrogue.co