Unveiling The Secrets Of Molar Mass N2: A Comprehensive Guide For Science Enthusiasts
Alright, let’s dive into the world of chemistry and uncover the mystery behind molar mass N2! If you’re a student, a science enthusiast, or just someone who wants to understand the basics of chemistry, you’re in the right place. Molar mass N2 is a term that might sound intimidating, but trust me, by the end of this article, you’ll be a pro at it. So, buckle up and let’s get started!
Now, you might be wondering, why should I care about molar mass N2? Well, understanding this concept is crucial if you want to grasp the basics of chemical reactions, gas laws, and even the behavior of molecules in our atmosphere. It’s like the foundation of a house—without it, everything else falls apart. So, let’s break it down step by step and make it super easy for you to digest.
In this article, we’ll explore everything you need to know about molar mass N2. From the basics to advanced applications, we’ve got you covered. Whether you’re preparing for an exam, working on a science project, or just curious about the world around you, this guide will provide you with all the information you need. Let’s roll!
- Unveiling The Power Of Wwwnothing2hide Salesforce Your Ultimate Guide
- Sophie Barker Missing Nyc The Mysterious Case Thats Left Everyone Guessing
What is Molar Mass N2 Anyway?
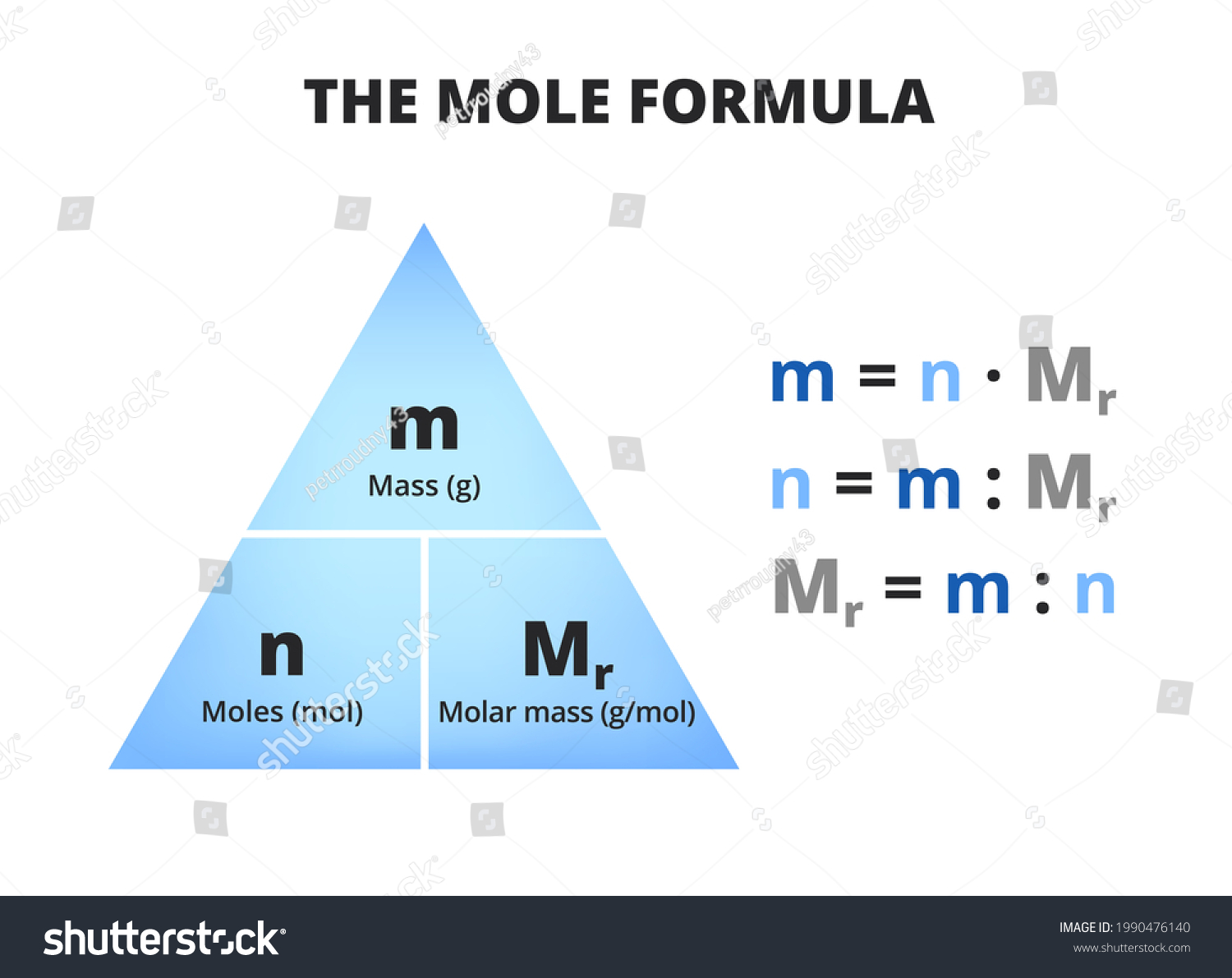
Let’s start with the basics. Molar mass N2 refers to the mass of one mole of nitrogen gas (N2). In simple terms, it’s the weight of 6.022 x 10^23 molecules of nitrogen gas. Yeah, that’s a lot of molecules! But don’t worry, we’ll break it down further so it’s easier to understand.
The molar mass of nitrogen gas is approximately 28.01 grams per mole. This value comes from the fact that nitrogen gas is made up of two nitrogen atoms, and each nitrogen atom has an atomic mass of about 14.007 atomic mass units (amu). So, when you add them together, you get the molar mass of N2.
Now, why is this important? Well, knowing the molar mass of a substance allows scientists to calculate the amount of a substance needed for a reaction, measure gas volumes, and even predict the behavior of gases under different conditions. Cool, right?
- Dan Schneider And Amanda Bynes The Dynamic Duo Behind Beloved Shows
- How Tall Is Tara Yummy Discover The Height And More About This Rising Star
Understanding the Basics of Molar Mass
Why Do We Need Molar Mass?
Molar mass is like a universal language in chemistry. It helps us compare different substances on a molecular level. For example, if you have one mole of nitrogen gas and one mole of oxygen gas, you can easily compare their weights because you know their molar masses. This is super useful in experiments, industrial processes, and even in everyday life.
Imagine you’re baking a cake and the recipe calls for a certain amount of sugar. You wouldn’t just throw in random amounts, right? The same goes for chemistry. Scientists need precise measurements, and molar mass helps them achieve that precision.
How to Calculate Molar Mass?
Calculating molar mass is pretty straightforward. All you need is a periodic table and a calculator. Here’s how you do it:
- Find the atomic mass of each element in the compound.
- Multiply the atomic mass by the number of atoms of that element in the compound.
- Add up all the values to get the molar mass of the compound.
For N2, since it’s made up of two nitrogen atoms, you simply multiply the atomic mass of nitrogen (14.007 amu) by 2. And voila, you get the molar mass of N2, which is approximately 28.01 grams per mole.
The Importance of Molar Mass in Chemistry
Molar mass plays a crucial role in various areas of chemistry. From stoichiometry to thermodynamics, it’s a concept that keeps popping up. Let’s explore some of its applications:
- Stoichiometry: Molar mass helps scientists calculate the amounts of reactants and products in a chemical reaction.
- Gas Laws: It’s used in calculations involving ideal gas laws, such as finding the volume of a gas at a given temperature and pressure.
- Concentration Calculations: Molar mass is essential when calculating the concentration of solutions in terms of molarity.
As you can see, molar mass is not just a theoretical concept—it has real-world applications that make it indispensable in the field of chemistry.
Applications of Molar Mass N2 in Real Life
Industrial Uses
Nitrogen gas (N2) is widely used in various industries. From food packaging to electronics manufacturing, its applications are vast. Understanding its molar mass helps industries optimize their processes and ensure safety. For example, nitrogen is used to displace oxygen in food packaging to prevent spoilage. Knowing the exact amount of nitrogen needed is crucial for maintaining product quality.
Environmental Impact
Nitrogen is a major component of the Earth’s atmosphere, making up about 78% of it. Its molar mass plays a role in understanding atmospheric chemistry and the behavior of gases in the environment. Scientists use this information to study climate change, air pollution, and other environmental issues.
Fun Facts About Molar Mass N2
Did you know that nitrogen gas is colorless, odorless, and tasteless? It’s also non-toxic and inert under standard conditions. These properties make it ideal for various applications. Here are some fun facts about molar mass N2:
- The molar mass of N2 is approximately 28.01 grams per mole, which is slightly heavier than air.
- Nitrogen is the most abundant element in the Earth’s atmosphere.
- It’s used in the production of ammonia, which is a key ingredient in fertilizers.
Isn’t it fascinating how something as simple as molar mass can have such a big impact on our lives?
Common Misconceptions About Molar Mass
There are a few misconceptions about molar mass that we need to clear up. For example, some people think that molar mass is the same as molecular weight. While they are related, they are not exactly the same. Molar mass refers to the mass of one mole of a substance, while molecular weight is the mass of a single molecule.
Another common misconception is that molar mass is only used in chemistry. In reality, it’s used in physics, biology, and even engineering. So, don’t limit your understanding of molar mass to just one field.
Advanced Concepts in Molar Mass N2
Isotopes and Their Effect on Molar Mass
Nitrogen has two stable isotopes: nitrogen-14 and nitrogen-15. The molar mass of N2 takes into account the natural abundance of these isotopes. Most nitrogen atoms are nitrogen-14, which is why the molar mass of N2 is approximately 28.01 grams per mole.
Molar Mass in Quantum Chemistry
In advanced chemistry, molar mass plays a role in quantum calculations. It’s used to determine the energy levels of molecules and predict their behavior under different conditions. This is particularly important in fields like spectroscopy and computational chemistry.
How to Use Molar Mass N2 in Experiments
Now that you know what molar mass N2 is and why it’s important, let’s talk about how to use it in experiments. Here are a few tips:
- Stoichiometric Calculations: Use molar mass to calculate the amounts of reactants and products in a reaction.
- Gas Volume Calculations: Use the ideal gas law to find the volume of nitrogen gas at a given temperature and pressure.
- Solution Preparation: Use molar mass to prepare solutions of known concentration.
Remember, accuracy is key in experiments. Always double-check your calculations and use reliable equipment to ensure precise measurements.
Conclusion
In conclusion, molar mass N2 is a fundamental concept in chemistry that has numerous applications in science and industry. From understanding the basics to exploring advanced topics, molar mass plays a vital role in our understanding of the world around us.
So, whether you’re a student, a scientist, or just someone who’s curious about chemistry, I hope this article has helped you understand the importance of molar mass N2. Now, it’s your turn to take action. Leave a comment below and let me know what you think. Or, if you found this article helpful, share it with your friends and colleagues. Together, let’s spread the love for science!
Table of Contents
- What is Molar Mass N2 Anyway?
- Understanding the Basics of Molar Mass
- The Importance of Molar Mass in Chemistry
- Applications of Molar Mass N2 in Real Life
- Fun Facts About Molar Mass N2
- Common Misconceptions About Molar Mass
- Advanced Concepts in Molar Mass N2
- How to Use Molar Mass N2 in Experiments
- Conclusion
- Young Paradise Login And Password A Beginners Guide To Unlocking Your Digital Paradise
- How To Defeat 2 Types Of Primal Constructs A Comprehensive Guide For Adventurers
3,595 Molar Mass Images, Stock Photos & Vectors Shutterstock

How do you Calculate the Molar Mass of a Substance? Write A Topic

The Mole Molar Mass And Molar Volume vrogue.co