Molar Mass Of N: Understanding Its Importance And Applications
When it comes to chemistry, the molar mass of N is one of those fundamental concepts that can make your head spin—but trust me, it’s not as complicated as it seems. If you’re diving into the world of chemical reactions and calculations, knowing the molar mass of nitrogen (N) is like having a secret weapon in your toolkit. Whether you’re a student trying to ace your exams or a science enthusiast looking to expand your knowledge, this article will break it down for you in simple terms. So, buckle up and let’s dive in!
Chemistry might feel like a foreign language at first, but once you grasp the basics, it becomes second nature. The molar mass of N is one of those foundational concepts that opens the door to understanding more complex topics. In this article, we’ll explore what molar mass is, why it matters, and how to calculate it for nitrogen. By the end, you’ll be equipped with the knowledge to tackle any related problem with confidence.
Let’s face it—chemistry isn’t everyone’s cup of tea. But if you want to ace your exams or impress your friends with your scientific know-how, understanding the molar mass of N is a great place to start. This concept is crucial for anyone working with nitrogen or studying chemistry in general. So, without further ado, let’s get started!
- Jonathan Roumie Wife The Fascinating Life Behind The Scenes
- Adrian Paul The Iconic Warrior And His Legacy
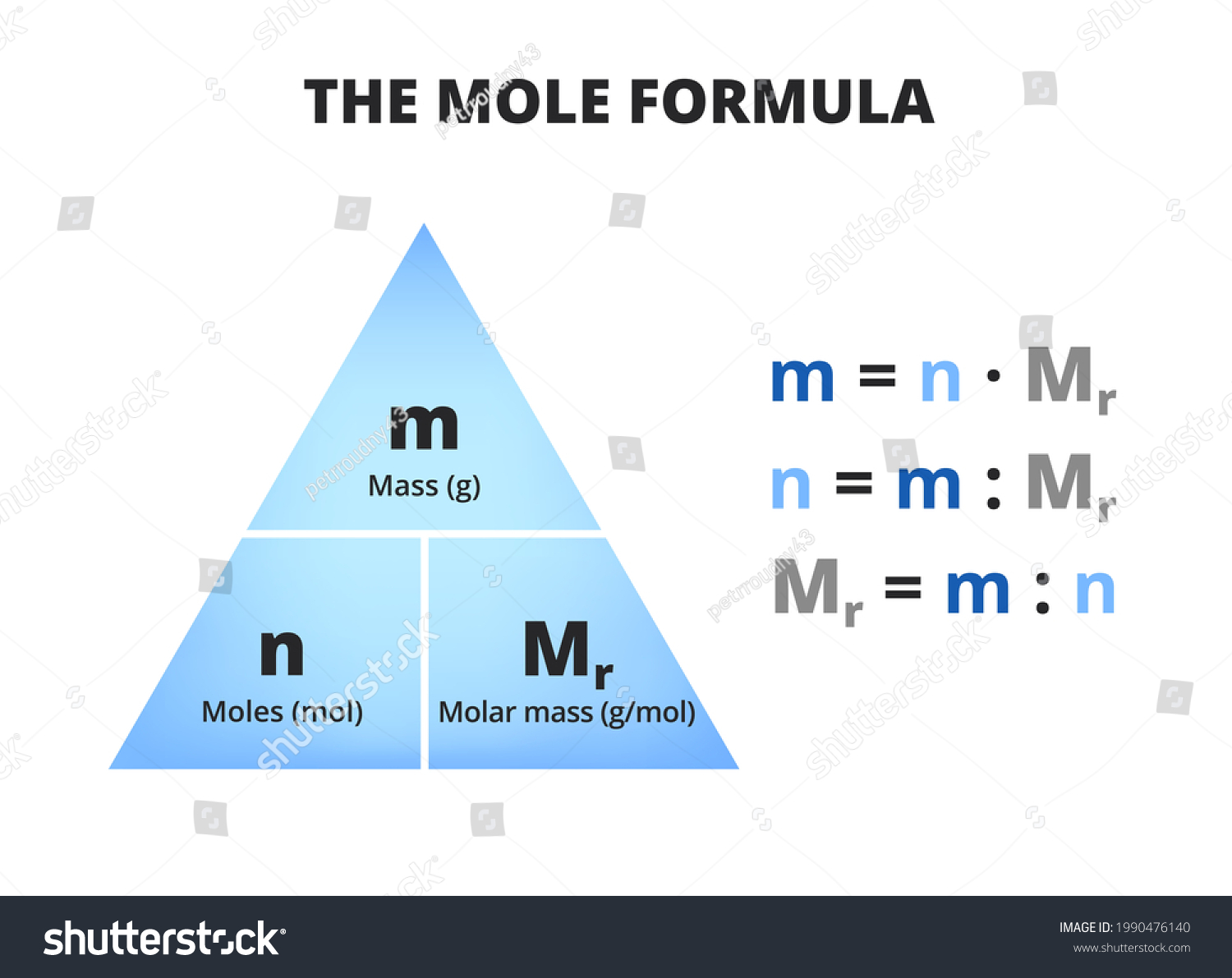
What is Molar Mass?
Molar mass is basically the weight of one mole of a substance. Think of it as the “weight” of a chemical element or compound in grams per mole (g/mol). It’s like weighing an atom or molecule on a super tiny scale. For nitrogen (N), the molar mass is approximately 14.01 g/mol. This value comes from the atomic mass of nitrogen, which is listed on the periodic table.
In simple terms, molar mass helps us bridge the gap between the microscopic world of atoms and the macroscopic world of grams and kilograms. It’s a crucial tool for chemists because it allows them to calculate the amount of a substance needed for reactions. Without molar mass, chemistry would be a lot more chaotic!
Why Does Molar Mass Matter?
Here’s the deal: molar mass is essential for calculating how much of a substance you need in a chemical reaction. Whether you’re working in a lab or solving problems in class, knowing the molar mass of N helps you determine the correct proportions. For example, if you’re mixing nitrogen with other elements, you’ll need to know how much nitrogen to add to achieve the desired result.
- Johnny Cade The Outsider Who Shaped A Generation
- Tim Walz Height The Inside Scoop Youve Been Waiting For
It also plays a role in real-world applications, such as manufacturing fertilizers, producing ammonia, and even understanding the composition of the Earth’s atmosphere. So, while molar mass might seem like a theoretical concept, it has some pretty practical uses!
Understanding the Molar Mass of N
Now that we’ve covered the basics of molar mass, let’s zoom in on nitrogen (N). Nitrogen is a fascinating element—it makes up about 78% of the Earth’s atmosphere and is vital for life as we know it. The molar mass of N is approximately 14.01 g/mol, which means one mole of nitrogen atoms weighs 14.01 grams.
But wait—there’s more! Nitrogen often exists as N₂ (diatomic nitrogen), which means two nitrogen atoms bonded together. In this case, the molar mass of N₂ is roughly 28.02 g/mol because you’re dealing with two nitrogen atoms instead of one. This distinction is important when working with nitrogen in chemical reactions.
How to Calculate the Molar Mass of N
Calculating the molar mass of N is pretty straightforward. You just need to look at the periodic table and find the atomic mass of nitrogen, which is 14.01. That’s it! If you’re dealing with N₂, you simply multiply the atomic mass by 2:
- Atomic mass of N = 14.01
- Molar mass of N₂ = 14.01 × 2 = 28.02 g/mol
See? It’s not rocket science (well, maybe a little). This simple calculation is the foundation for more complex chemistry problems, so it’s worth mastering early on.
Applications of the Molar Mass of N
So, why should you care about the molar mass of N? Besides being a key concept in chemistry, it has some pretty cool real-world applications. Here are a few examples:
- Fertilizer Production: Nitrogen is a crucial component of fertilizers, which help plants grow. By understanding the molar mass of N, scientists can calculate the exact amount of nitrogen needed for different types of fertilizers.
- Ammonia Synthesis: Ammonia (NH₃) is produced using nitrogen and hydrogen in a process called the Haber-Bosch process. Knowing the molar mass of N helps chemists optimize this reaction.
- Atmospheric Studies: Since nitrogen makes up most of the Earth’s atmosphere, understanding its molar mass is essential for studying air composition and climate change.
These applications show just how important the molar mass of N is in both laboratory settings and everyday life. Whether you’re a scientist, engineer, or just someone curious about the world, this concept has something to offer.
Real-World Examples
Let’s look at a real-world example to see how the molar mass of N is used. Imagine you’re working in a fertilizer factory and need to produce 100 grams of ammonium nitrate (NH₄NO₃). To do this, you’ll need to calculate the amount of nitrogen required based on its molar mass.
Here’s how you’d do it:
- Molar mass of NH₄NO₃ = 80.05 g/mol
- Molar mass of N in NH₄NO₃ = 2 × 14.01 = 28.02 g/mol
- Percentage of N in NH₄NO₃ = (28.02 ÷ 80.05) × 100 = 35.01%
So, to produce 100 grams of ammonium nitrate, you’ll need about 35.01 grams of nitrogen. Simple, right?
Common Misconceptions About Molar Mass
There are a few common misconceptions about molar mass that can trip people up. Let’s clear them up:
- Molar Mass vs. Atomic Mass: Some people confuse molar mass with atomic mass. While they’re related, they’re not the same thing. Atomic mass refers to the mass of a single atom, while molar mass refers to the mass of one mole of atoms.
- Diatomic vs. Monatomic: Remember, nitrogen often exists as N₂, so its molar mass is different from that of a single nitrogen atom. Always check whether you’re dealing with N or N₂ in a reaction.
- Units Matter: Molar mass is always expressed in grams per mole (g/mol). If you forget the units, your calculations might be off.
By avoiding these common pitfalls, you’ll be able to calculate molar mass accurately and confidently.
Why Units Are Important
Units are like the secret sauce in chemistry—they make everything work. If you’re calculating molar mass and forget to include the units, your answer won’t make sense. Always double-check that you’re using grams per mole (g/mol) when working with molar mass. It’s a small detail, but it can make a big difference!
How to Use Molar Mass in Chemical Reactions
Now that you know what molar mass is and how to calculate it, let’s talk about how to use it in chemical reactions. This is where things get interesting! Molar mass helps you determine the stoichiometry of a reaction, which is just a fancy way of saying how much of each reactant you need.
Here’s a step-by-step guide:
- Identify the balanced chemical equation.
- Find the molar mass of each reactant and product.
- Use the molar mass to calculate the mass of each substance needed.
For example, let’s say you’re working with the reaction N₂ + 3H₂ → 2NH₃. To calculate how much nitrogen you need to produce 50 grams of ammonia, you’d use the molar mass of N₂ and NH₃. Trust me, it’s easier than it sounds!
Stoichiometry Made Simple
Stoichiometry might sound intimidating, but it’s really just about balancing equations and using molar mass to figure out how much of each substance you need. By mastering this skill, you’ll be able to tackle even the most complex chemical reactions with ease.
Advanced Concepts: Beyond Molar Mass
Once you’ve got the basics of molar mass down, you can start exploring more advanced concepts. For example, you might want to dive into molecular weight, formula weight, or even molar volume. These concepts build on the foundation of molar mass and open up new possibilities for understanding chemistry.
Here are a few advanced topics to consider:
- Molecular Weight: Similar to molar mass, but specifically for molecules rather than individual atoms.
- Formula Weight: Used for ionic compounds, this is the sum of the atomic weights of all the atoms in a formula unit.
- Molar Volume: The volume occupied by one mole of a substance, usually expressed in liters per mole (L/mol).
These concepts might seem overwhelming at first, but with practice, they’ll become second nature. And trust me, they’re worth the effort!
Why Advanced Concepts Matter
Understanding advanced concepts like molecular weight and molar volume can give you a deeper appreciation for chemistry. It’s like unlocking new levels in a video game—each concept builds on the last, giving you more tools to solve complex problems. So, don’t be afraid to dive in and explore!
Conclusion: Why Molar Mass of N Matters
In conclusion, the molar mass of N is a fundamental concept in chemistry that has far-reaching implications. From calculating the amount of nitrogen needed for chemical reactions to understanding the composition of the Earth’s atmosphere, molar mass plays a crucial role in both theoretical and practical applications.
By mastering this concept, you’ll be well-equipped to tackle more complex chemistry problems and gain a deeper understanding of the world around you. So, whether you’re a student, scientist, or curious learner, the molar mass of N is definitely worth your time and attention.
Now it’s your turn! Leave a comment below and let me know what you think about the molar mass of N. Have you ever used it in a real-world application? Share your experiences and let’s keep the conversation going. And if you found this article helpful, don’t forget to share it with your friends and fellow chemistry enthusiasts!
Daftar Isi
- What is Molar Mass?
- Why Does Molar Mass Matter?
- Understanding the Molar Mass of N
- How to Calculate the Molar Mass of N
- Applications of the Molar Mass of N
- Common Misconceptions About Molar Mass
- How to Use Molar Mass in Chemical Reactions
- Advanced Concepts: Beyond Molar Mass
- Why Advanced Concepts Matter
- Conclusion: Why Molar Mass of N Matters
- Park And Recreation Cast The Ultimate Fan Guide To Your Favorite Pawnee Stars
- 59 Million Unpacking The Dannielynn Birkhead Net Worth Saga
3,595 Molar Mass Images, Stock Photos & Vectors Shutterstock

Molar mass of al dinojord

How do you Calculate the Molar Mass of a Substance? Write A Topic