Nitrogen G/mol: Understanding The Basics And Beyond
Alright, let's dive right into the world of nitrogen g/mol! If you're here, chances are you're either a science enthusiast, a student trying to ace your chemistry exam, or someone who just wants to understand what all this fuss is about. Nitrogen g/mol is a concept that might sound intimidating at first, but trust me, by the end of this article, you'll have it down pat. So, buckle up and let's get started.
Nitrogen g/mol, or the molar mass of nitrogen, is essentially the weight of one mole of nitrogen atoms. Now, before you freak out, let me break it down for you. A mole is like a fancy way of counting atoms or molecules. Think of it as a "chemist's dozen," but instead of 12, it's a really big number called Avogadro's number. So, when we talk about nitrogen g/mol, we're just figuring out how much a bunch of nitrogen atoms weigh.
Why does this matter, you ask? Well, nitrogen is everywhere! It makes up about 78% of the air we breathe, and it plays a crucial role in everything from fertilizers to explosives. Understanding its molar mass helps scientists and engineers figure out how much nitrogen they need for different applications. Whether you're mixing chemicals in a lab or designing a rocket, knowing the molar mass of nitrogen is a pretty big deal.
- Barron Trump And Animal Cruelty A Closer Look At The Controversy
- Primed Deadly Efficiency The Ultimate Guide To Mastering Precision And Power
What Exactly is Nitrogen g/mol?
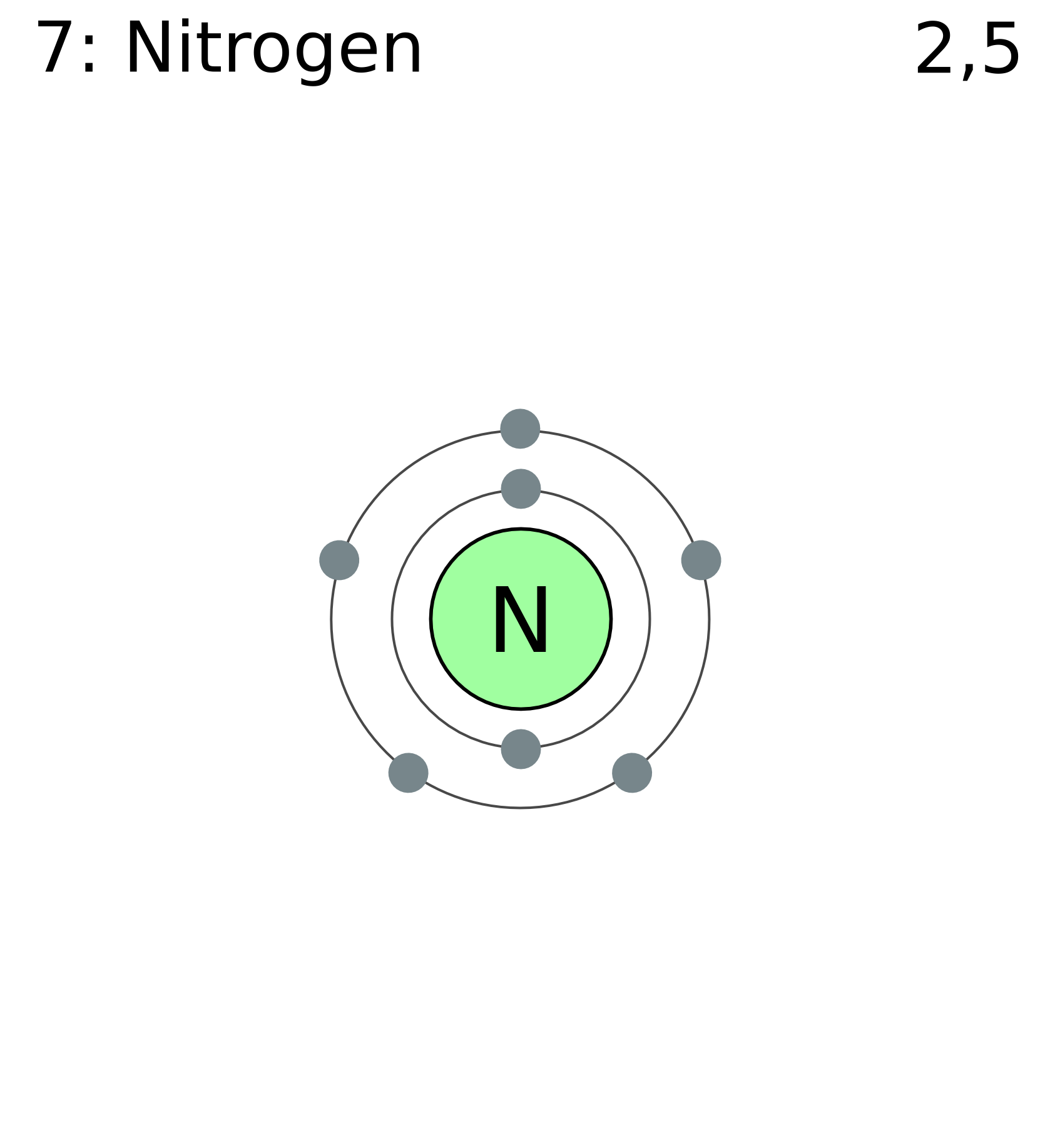
Let's get into the nitty-gritty. Nitrogen g/mol refers to the mass of one mole of nitrogen atoms, expressed in grams per mole (g/mol). For nitrogen, this number is approximately 14.01 g/mol. This means that if you had a mole of nitrogen atoms, they'd weigh about 14.01 grams. Pretty neat, right?
Now, here's the cool part: nitrogen atoms don't usually hang out alone. In nature, nitrogen is mostly found as N2, which means two nitrogen atoms are bonded together. So, if you're dealing with nitrogen gas (N2), the molar mass doubles to around 28.02 g/mol. It's like nitrogen has a little buddy, and together they weigh twice as much!
Why is Nitrogen g/mol Important?
Nitrogen g/mol is more than just a number on a periodic table. It's a key piece of information that helps scientists and engineers do their jobs. For instance, in agriculture, knowing the molar mass of nitrogen helps farmers calculate how much fertilizer they need to apply to their crops. Too little nitrogen, and the plants won't grow. Too much, and you risk polluting the environment.
- African Mammal With Striped Legs Discovering Naturersquos Hidden Gems
- Off Grid Misty Age The Ultimate Guide To Embracing A Sustainable Future
In the world of chemistry, nitrogen g/mol is crucial for stoichiometry, which is basically the math of chemical reactions. By knowing the molar mass of nitrogen, chemists can figure out how much of one substance they need to react with another. It's like following a recipe, but instead of flour and sugar, you're dealing with atoms and molecules.
How is Nitrogen g/mol Calculated?
Calculating nitrogen g/mol is surprisingly straightforward. You just need to look at the periodic table. The atomic mass of nitrogen is listed as approximately 14.01 atomic mass units (amu). Since one mole of nitrogen atoms weighs the same as its atomic mass in grams, nitrogen g/mol is simply 14.01 g/mol.
For nitrogen gas (N2), you just multiply this number by two because there are two nitrogen atoms in each molecule. So, the molar mass of N2 is about 28.02 g/mol. Easy peasy!
Steps to Calculate Nitrogen g/mol:
- Find the atomic mass of nitrogen on the periodic table (14.01 amu).
- Convert this to grams per mole (14.01 g/mol).
- If you're dealing with N2, multiply by two (28.02 g/mol).
Applications of Nitrogen g/mol
Nitrogen g/mol isn't just a theoretical concept; it has real-world applications. Here are a few examples:
Agriculture
In agriculture, nitrogen is a vital nutrient for plant growth. Fertilizers are often enriched with nitrogen to help crops thrive. By knowing the molar mass of nitrogen, farmers can calculate how much fertilizer to use, ensuring their crops get the right amount of nutrients without wasting resources or harming the environment.
Industry
In the industrial sector, nitrogen is used in a variety of applications, from making ammonia for fertilizers to producing nitric acid for explosives. Understanding nitrogen g/mol helps engineers optimize these processes, ensuring efficiency and safety.
Environmental Science
Nitrogen g/mol also plays a role in environmental science. Scientists use it to study nitrogen cycles in ecosystems and to monitor nitrogen pollution in water and air. By tracking nitrogen levels, they can develop strategies to protect our planet's health.
Common Misconceptions About Nitrogen g/mol
There are a few common misconceptions about nitrogen g/mol that we need to clear up. First, some people think that nitrogen g/mol is the same for all forms of nitrogen. Not true! While the molar mass of a single nitrogen atom is 14.01 g/mol, nitrogen gas (N2) has a molar mass of 28.02 g/mol because it consists of two nitrogen atoms.
Another misconception is that nitrogen g/mol is only relevant to chemists. In reality, it has applications in fields as diverse as agriculture, medicine, and environmental science. Whether you're a farmer, a doctor, or an environmentalist, understanding nitrogen g/mol can be incredibly useful.
Fun Facts About Nitrogen
Here are a few fun facts about nitrogen to brighten your day:
- Nitrogen makes up about 78% of the Earth's atmosphere.
- It's colorless, odorless, and tasteless.
- Nitrogen is essential for life, as it's a key component of DNA, RNA, and proteins.
- The word "nitrogen" comes from the Greek words "nitron" (sodium carbonate) and "genes" (forming).
Challenges in Working with Nitrogen g/mol
While nitrogen g/mol is a useful concept, there are challenges in working with it. One of the biggest challenges is ensuring accuracy in calculations. Even small errors in molar mass can lead to big problems in chemical reactions or industrial processes. That's why it's crucial to double-check your numbers and use reliable sources.
Another challenge is dealing with different forms of nitrogen. As we've seen, the molar mass of nitrogen changes depending on whether you're dealing with individual atoms, N2 gas, or nitrogen compounds. Keeping track of these differences can be tricky, but it's essential for getting accurate results.
Future Developments in Nitrogen Research
The study of nitrogen g/mol and its applications is an ongoing field of research. Scientists are constantly exploring new ways to use nitrogen in agriculture, industry, and environmental science. For example, researchers are developing more efficient nitrogen fertilizers that reduce environmental impact. They're also studying how nitrogen cycles through ecosystems and how climate change might affect these cycles.
As our understanding of nitrogen g/mol grows, so too will its applications. Who knows? Maybe one day we'll find a way to harness nitrogen's power to solve some of the world's biggest challenges, from food security to climate change.
Emerging Technologies
Some of the most exciting developments in nitrogen research involve emerging technologies. For instance, scientists are exploring ways to capture and recycle nitrogen from waste streams, turning what was once pollution into a valuable resource. They're also developing new materials that can store nitrogen more efficiently, paving the way for cleaner energy solutions.
Conclusion
So, there you have it! Nitrogen g/mol might sound like a mouthful, but it's a concept that's both fascinating and incredibly useful. Whether you're a student, a scientist, or just someone who's curious about the world around you, understanding nitrogen g/mol can open up a whole new world of possibilities.
Now, here's the deal: if you found this article helpful, I'd love for you to share it with your friends and family. Knowledge is power, and the more people who understand concepts like nitrogen g/mol, the better off we'll all be. So, go ahead and hit that share button, leave a comment, or check out some of our other articles. Trust me, you won't regret it!
- 50 Meters To Ft The Ultimate Conversion Guide Youve Been Searching For
- Unlocking The Secrets Of Surveyspanoramaedkipp Texaslogin A Comprehensive Guide
Nitrogen Generators How It Works CGT
Collection of Nitrogen PNG. PlusPNG
Collection of Nitrogen PNG. PlusPNG