Nitrogen Molecular Mass: Everything You Need To Know
Let’s face it, folks—chemistry can sometimes feel like a foreign language. But today, we’re diving deep into one of the most essential elements in the universe: nitrogen. Specifically, we’re cracking the code on nitrogen molecular mass. Whether you’re a student trying to ace your chemistry exam, a science enthusiast, or just someone curious about the building blocks of life, this article is for you. So buckle up, because we’re about to break it down in a way that even your grandma could understand.
Now, let’s get one thing straight: nitrogen isn’t just some random element hanging out on the periodic table. It’s everywhere! From the air we breathe to the DNA in our cells, nitrogen plays a starring role in the grand theater of life. And when we talk about nitrogen molecular mass, we’re essentially talking about how much “stuff” is packed into a molecule of nitrogen gas (N₂). Trust me, it’s more fascinating than it sounds.
Before we dive headfirst into the nitty-gritty, here’s a quick heads-up: this article is packed with juicy facts, easy-to-understand explanations, and even a few fun tidbits to keep things interesting. So whether you’re here for the science or just looking for a good time, you’re in the right place. Let’s go!
- Unveiling The Power Of Wwwnothing2hide Salesforce Your Ultimate Guide
- Floriana Lima The Rising Star Whos Capturing Hearts Worldwide
What Exactly is Nitrogen Molecular Mass?
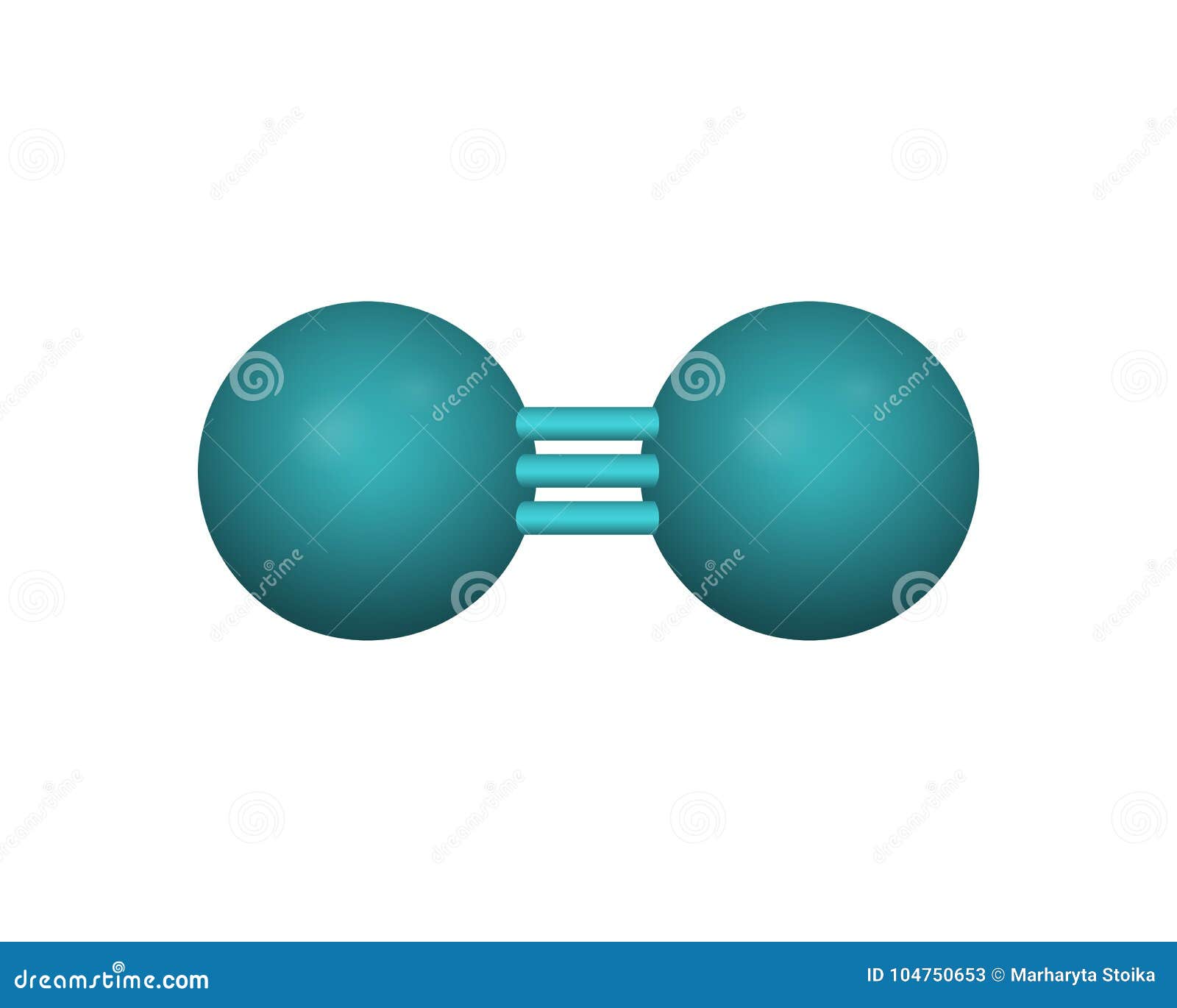
Alright, let’s start with the basics. Nitrogen molecular mass refers to the combined weight of the atoms in a nitrogen molecule. In the case of nitrogen gas (N₂), we’re talking about two nitrogen atoms bonded together. Think of it like a dynamic duo—each atom has its own weight, and when they team up, their weights add up to form the molecular mass.
Now, you might be wondering, “Why does this matter?” Well, my friend, understanding molecular mass is crucial in chemistry because it helps us figure out how much of a substance we’re dealing with. For example, if you’re working in a lab and need to measure out a precise amount of nitrogen gas, knowing its molecular mass is key.
So, what’s the big number? Drumroll, please… The molecular mass of nitrogen gas (N₂) is approximately 28.014 atomic mass units (amu). That’s the sum of the atomic masses of the two nitrogen atoms in the molecule. Easy peasy, right?
- Tara Yummy Height Discovering The Real Stats And More
- Ronald Fenty The Man Behind The Music And Fashion Empire
Why is Nitrogen So Important?
Let’s take a step back and appreciate why nitrogen deserves all the attention. Nitrogen makes up about 78% of the Earth’s atmosphere, making it the most abundant gas in the air we breathe. But its importance doesn’t stop there. Nitrogen is also a critical component of amino acids, which are the building blocks of proteins, and nucleic acids, which include DNA and RNA. In short, life as we know it wouldn’t exist without nitrogen.
Here’s a fun fact: nitrogen is kind of like the ultimate multitasker. It’s used in everything from fertilizers to explosives to cryogenic freezing. And let’s not forget its role in the nitrogen cycle, which keeps ecosystems running smoothly. Pretty impressive for an element that’s invisible to the naked eye, huh?
Breaking Down the Chemistry: Nitrogen Atoms and Bonds
Now that we’ve established why nitrogen is so important, let’s zoom in on the molecular level. A nitrogen atom has an atomic number of 7, which means it has 7 protons in its nucleus. Its atomic mass is approximately 14.007 amu, which is a little less than half of the molecular mass of N₂.
When two nitrogen atoms come together to form N₂, they create a triple bond. This triple bond is super strong, making nitrogen gas one of the most stable molecules out there. It’s like a fortress—almost nothing can break it apart unless you apply a ton of energy. This stability is one of the reasons why nitrogen gas is so abundant in the atmosphere.
Why the Triple Bond Matters
Let’s talk about why the triple bond is such a big deal. First off, it’s what gives nitrogen gas its incredible stability. The bond is so strong that it takes a lot of energy to break it apart, which is why nitrogen gas is often used as an inert gas in industrial applications.
But here’s the kicker: when you do manage to break that triple bond, you unleash a ton of energy. This is why nitrogen is used in explosives and propellants. Think about it—something so stable can also be so powerful when unleashed. Nature is wild like that!
How is Nitrogen Molecular Mass Calculated?
Calculating the molecular mass of nitrogen gas (N₂) is actually pretty straightforward. All you need to do is add up the atomic masses of the two nitrogen atoms in the molecule. Each nitrogen atom has an atomic mass of approximately 14.007 amu, so:
Nitrogen molecular mass = 14.007 amu + 14.007 amu = 28.014 amu
Simple, right? But here’s where things get interesting: in real-world scenarios, the molecular mass of nitrogen gas might vary slightly due to isotopes. Isotopes are variations of an element that have the same number of protons but different numbers of neutrons. While nitrogen-14 is the most common isotope, nitrogen-15 also exists in small amounts, which can affect the average molecular mass.
Isotopes: The Wildcards of Chemistry
Isotopes might sound complicated, but they’re actually pretty cool. Think of them like siblings in a family—same parents, different quirks. In the case of nitrogen, nitrogen-14 and nitrogen-15 are like fraternal twins. They’re both nitrogen, but they have slightly different weights due to the extra neutron in nitrogen-15.
This variation is why the molecular mass of nitrogen gas isn’t exactly 28.000 amu. Instead, it’s 28.014 amu, which takes into account the natural abundance of both isotopes. Science is all about precision, folks!
Applications of Nitrogen in Real Life
Now that we’ve covered the basics, let’s talk about how nitrogen is used in everyday life. Nitrogen might be invisible, but its impact is everywhere. Here are just a few examples:
- Fertilizers: Nitrogen is a key ingredient in fertilizers, helping plants grow strong and healthy.
- Food Preservation: Nitrogen gas is often used to displace oxygen in food packaging, extending shelf life and preventing spoilage.
- Industrial Applications: Nitrogen gas is used in everything from electronics manufacturing to tire inflation.
- Cryogenics: Liquid nitrogen is used to freeze materials and even preserve biological samples.
As you can see, nitrogen is a versatile little element with a ton of practical applications. And it all starts with understanding its molecular mass and properties.
Environmental Impact of Nitrogen
While nitrogen is essential for life, it’s not without its downsides. Excessive use of nitrogen-based fertilizers can lead to environmental problems like water pollution and algal blooms. These issues occur when excess nitrogen makes its way into waterways, causing an overgrowth of algae that can deplete oxygen levels and harm aquatic life.
But here’s the good news: scientists and engineers are working hard to develop sustainable practices that minimize nitrogen’s negative impact. By using nitrogen more efficiently and developing alternative fertilizers, we can strike a balance between feeding the world and protecting the planet.
The Nitrogen Cycle: Nature’s Recycling System
The nitrogen cycle is one of nature’s most brilliant recycling systems. It’s a complex process that involves the movement of nitrogen through the atmosphere, biosphere, and geosphere. Here’s a quick rundown:
- Nitrogen Fixation: Certain bacteria convert atmospheric nitrogen into a form that plants can use.
- Nitrification: Bacteria convert ammonia into nitrates, which plants can absorb.
- Denitrification: Bacteria convert nitrates back into nitrogen gas, completing the cycle.
Understanding the nitrogen cycle is crucial for managing nitrogen’s impact on the environment. It’s a delicate balance, but one that’s essential for maintaining healthy ecosystems.
Fun Facts About Nitrogen
Let’s wrap up with some fun facts about nitrogen to impress your friends at your next dinner party:
- Nitrogen gas is colorless, odorless, and tasteless.
- It’s used in the production of nitrous oxide, aka laughing gas.
- Nitrogen is the sixth most abundant element in the universe.
- It’s possible to freeze biological samples using liquid nitrogen without damaging them.
Who knew nitrogen could be so fascinating? It’s truly one of the unsung heroes of the periodic table.
Conclusion: Why Nitrogen Matters
So there you have it—a deep dive into nitrogen molecular mass and everything that makes nitrogen such an incredible element. From its role in the atmosphere to its applications in science and industry, nitrogen is truly a force to be reckoned with. And while its molecular mass might seem like a small detail, it’s a crucial piece of the puzzle when it comes to understanding this amazing element.
Now it’s your turn! Whether you’re a student, a science enthusiast, or just someone curious about the world around you, I encourage you to share this article with your friends and family. Who knows? You might just spark a conversation that changes someone’s perspective on chemistry. And if you’re hungry for more science goodness, be sure to check out our other articles on the wonders of the periodic table.
Thanks for joining me on this journey into the world of nitrogen. Until next time, stay curious and keep learning!
Table of Contents
- What Exactly is Nitrogen Molecular Mass?
- Why is Nitrogen So Important?
- Breaking Down the Chemistry: Nitrogen Atoms and Bonds
- How is Nitrogen Molecular Mass Calculated?
- Applications of Nitrogen in Real Life
- Environmental Impact of Nitrogen
- Fun Facts About Nitrogen
- Conclusion: Why Nitrogen Matters
- Urban Medicine Tarkov Your Ultimate Guide To Surviving The Chaos
- Watchnixtoons2 Your Ultimate Destination For Anime Streaming

Nitrogen Atomic Number Mass Number Stock Illustration 1937507947
The Molecular Formula of Nitrogen. Stock Vector Illustration of

Nitrogen Periodic Table Diagram Nitrogen Atomic Mass vrogue.co