Unlocking The Secrets Of IR Cyclohexane: A Comprehensive Guide
When it comes to understanding organic chemistry, IR cyclohexane is one of those topics that can leave even the brightest minds scratching their heads. But don't worry, because we're about to break it down for you in a way that's easy to digest. Whether you're a student, researcher, or just someone curious about the world of molecules, this article has got you covered. Get ready to dive deep into the fascinating world of IR cyclohexane!
Picture this: you're in a lab, surrounded by flasks, beakers, and a bunch of other science-y stuff. You're trying to figure out what makes cyclohexane so special when it comes to infrared spectroscopy. Well, buckle up, because we're about to take you on a journey through the molecular landscape of this compound. From its structure to its vibrational frequencies, we've got all the details you need to know.
Now, before we get too deep into the nitty-gritty, let's talk about why IR cyclohexane is such a big deal. In the world of analytical chemistry, infrared spectroscopy is like a detective's best friend. It helps scientists identify unknown compounds and understand their behavior. And guess what? Cyclohexane plays a starring role in this scientific mystery. So, if you're ready to uncover the secrets behind this molecule, keep reading!
- Unveiling The Height Of Jack Nicholson A Deep Dive Into The Iconic Actors Stats
- How To Defeat 2 Types Of Primal Constructs A Comprehensive Guide For Adventurers
What is IR Cyclohexane?
Let's start with the basics. IR cyclohexane refers to the infrared spectrum of cyclohexane, a cyclic hydrocarbon with the molecular formula C6H12. This molecule is a key player in organic chemistry because of its unique properties. When you shine infrared light on cyclohexane, it absorbs certain wavelengths, creating a unique fingerprint that scientists can use to identify it. Cool, right?
But why does this matter? Well, understanding the IR spectrum of cyclohexane allows chemists to study its molecular vibrations and how they interact with light. This information is crucial for everything from drug development to environmental analysis. So, whether you're trying to create the next blockbuster medication or analyze pollutants in the air, IR cyclohexane has got your back.
Why Cyclohexane is Special
Now, you might be wondering, "What makes cyclohexane so special compared to other hydrocarbons?" Great question! Cyclohexane's ring structure gives it some unique properties that make it stand out in the world of chemistry. For one, it's more stable than its linear counterparts, which makes it less reactive and easier to work with in the lab.
- Tim Walz Height The Inside Scoop Youve Been Waiting For
- Homer James Jigme Gere The Fascinating Life Story Of A Modernday Legend
Additionally, cyclohexane's IR spectrum is relatively simple compared to other complex molecules. This simplicity makes it an excellent starting point for students and researchers who are just beginning to explore the world of infrared spectroscopy. So, if you're new to this field, cyclohexane is a great place to start your journey.
Understanding the Structure of Cyclohexane
To truly grasp the concept of IR cyclohexane, you need to understand its molecular structure. Cyclohexane is a six-membered ring made up of carbon atoms, each bonded to two hydrogen atoms. This ring can exist in two main conformations: chair and boat. The chair conformation is the most stable, which is why it's the one you'll most often encounter in the lab.
Now, here's where things get interesting. The chair conformation of cyclohexane has two types of hydrogen atoms: axial and equatorial. Axial hydrogens point straight up or down from the ring, while equatorial hydrogens are positioned more to the sides. These differences in orientation can affect how cyclohexane interacts with infrared light, giving it a distinctive IR spectrum.
The Importance of Molecular Conformations
Why do molecular conformations matter in IR spectroscopy? Well, the way a molecule is shaped can influence how it absorbs infrared light. In the case of cyclohexane, the chair conformation is particularly important because it's the most stable and therefore the most common form you'll encounter. By studying the IR spectrum of cyclohexane in its chair conformation, scientists can gain valuable insights into its molecular vibrations and how they relate to its structure.
How IR Spectroscopy Works
So, how exactly does IR spectroscopy work? When infrared light passes through a sample, certain wavelengths are absorbed by the molecules in the sample. These absorptions correspond to specific molecular vibrations, such as stretching and bending. By analyzing the pattern of absorptions, scientists can identify the types of bonds present in the molecule and even determine its structure.
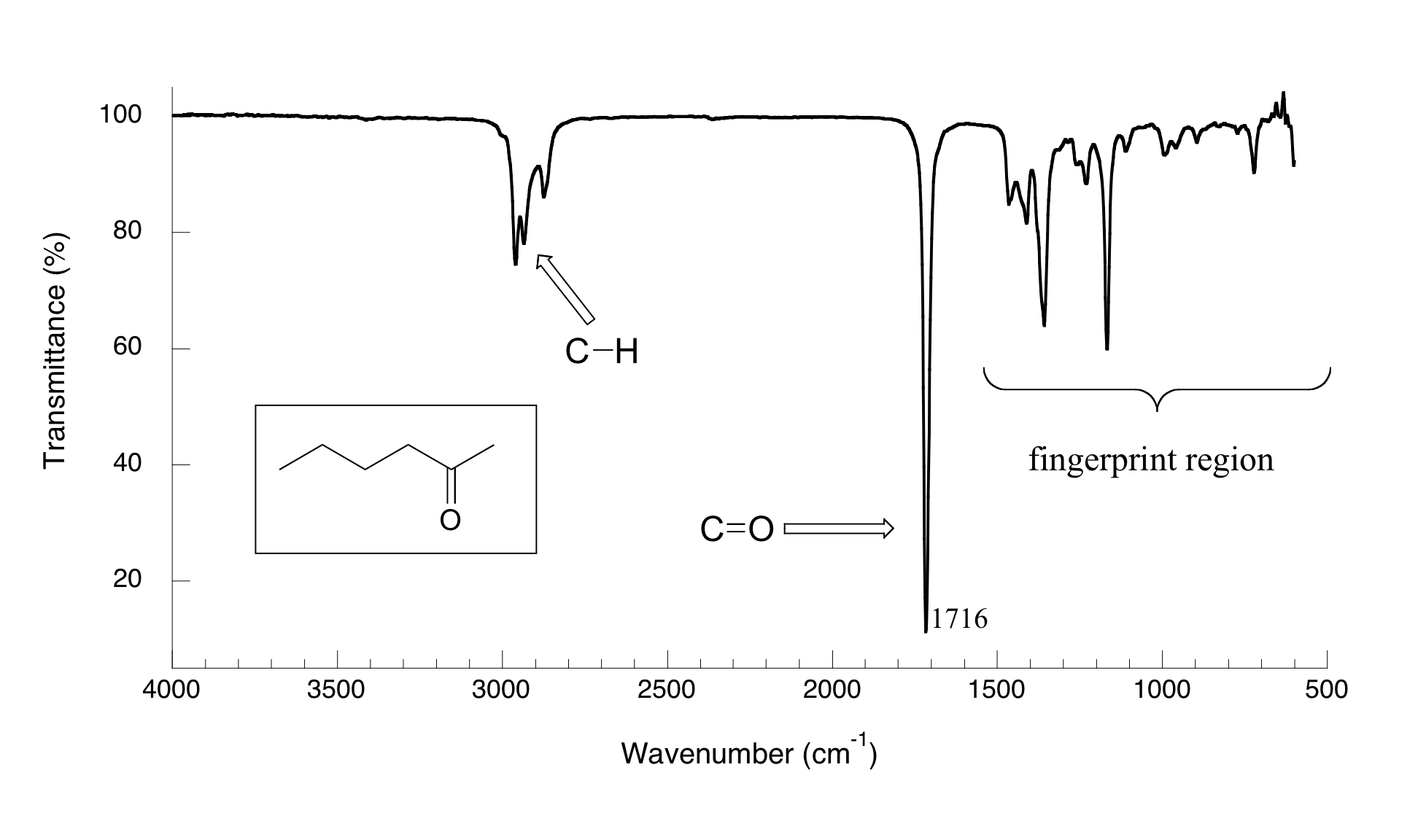
In the case of IR cyclohexane, the molecule absorbs infrared light at specific frequencies that correspond to its C-H stretching vibrations. These absorptions appear as peaks in the IR spectrum, and their positions and intensities can tell us a lot about the molecule's structure and behavior. Pretty cool, huh?
Key Peaks in the IR Spectrum of Cyclohexane
Let's take a closer look at the key peaks in the IR spectrum of cyclohexane. The most prominent peaks occur around 2850-3000 cm⁻¹, which correspond to the C-H stretching vibrations of the molecule. These peaks are broad and intense, making them easy to identify. Additionally, you might notice smaller peaks around 1450 cm⁻¹, which correspond to C-H bending vibrations.
By analyzing these peaks, scientists can confirm the presence of cyclohexane in a sample and even distinguish it from other hydrocarbons. This ability to identify and differentiate molecules is what makes IR spectroscopy such a powerful tool in the world of chemistry.
Applications of IR Cyclohexane
Now that we've covered the basics of IR cyclohexane, let's talk about its real-world applications. Cyclohexane is used in a wide range of industries, from pharmaceuticals to plastics. In each of these fields, understanding its IR spectrum can provide valuable insights into its behavior and interactions.
For example, in the pharmaceutical industry, IR spectroscopy is used to ensure the purity of cyclohexane and other solvents used in drug synthesis. In the environmental field, IR cyclohexane can help scientists detect and quantify pollutants in air and water samples. And in materials science, understanding the IR spectrum of cyclohexane can aid in the development of new polymers and coatings.
Real-World Examples of IR Cyclohexane in Action
Let's look at a few real-world examples of how IR cyclohexane is used in practice. In one study, researchers used IR spectroscopy to analyze the degradation of cyclohexane in the atmosphere. By monitoring the changes in its IR spectrum over time, they were able to gain insights into the molecule's reaction pathways and environmental impact.
In another example, pharmaceutical companies use IR spectroscopy to verify the identity and purity of cyclohexane used in the synthesis of active pharmaceutical ingredients. This ensures that the final product meets the necessary quality standards and is safe for use in humans.
Challenges in IR Cyclohexane Analysis
Of course, no scientific technique is without its challenges. When it comes to IR cyclohexane analysis, there are a few things to keep in mind. For one, the molecule's relatively simple IR spectrum can sometimes make it difficult to distinguish from other hydrocarbons. Additionally, the presence of impurities or other compounds in a sample can complicate the analysis.
To overcome these challenges, scientists often use advanced techniques such as Fourier-transform infrared (FTIR) spectroscopy, which provides higher resolution and more detailed information about the molecule's vibrational modes. By combining FTIR with other analytical techniques, researchers can gain a more complete understanding of cyclohexane's behavior and interactions.
Tips for Accurate IR Cyclohexane Analysis
Here are a few tips to help you get the most accurate results when analyzing IR cyclohexane:
- Use high-purity samples to minimize interference from impurities.
- Employ advanced techniques like FTIR for higher resolution and more detailed information.
- Compare your results to reference spectra to ensure accuracy and consistency.
- Consider using complementary techniques, such as NMR or mass spectrometry, for a more comprehensive analysis.
Conclusion
And there you have it, folks! A comprehensive guide to IR cyclohexane that covers everything from its molecular structure to its real-world applications. Whether you're a student, researcher, or just someone curious about the world of chemistry, we hope this article has given you a deeper understanding of this fascinating molecule.
So, what's next? If you've enjoyed this deep dive into IR cyclohexane, why not explore some of the other topics we've covered on our site? From organic chemistry to analytical techniques, we've got something for everyone. And don't forget to leave a comment or share this article with your friends and colleagues. After all, knowledge is meant to be shared!
Table of Contents
- What is IR Cyclohexane?
- Understanding the Structure of Cyclohexane
- How IR Spectroscopy Works
- Applications of IR Cyclohexane
- Challenges in IR Cyclohexane Analysis
- Conclusion
Remember, the world of chemistry is full of fascinating discoveries just waiting to be uncovered. So keep exploring, keep learning, and most importantly, keep asking questions. Happy analyzing!
- Www Freeworlder Org Your Ultimate Guide To Travel And Adventure
- Why The Arcyart Artist Directory Is Your Ultimate Creative Companion
Ir Frequency Table Amide

Cyclohexane, UVIR RCI LABSCAN LIMITED (EN)
Solved Below are the IR spectra for hexane. 1hexene. and